Colosafe®
Methylation Detection Kit for Human SDC2 Gene (Real time PCR) & Stool Collection Device
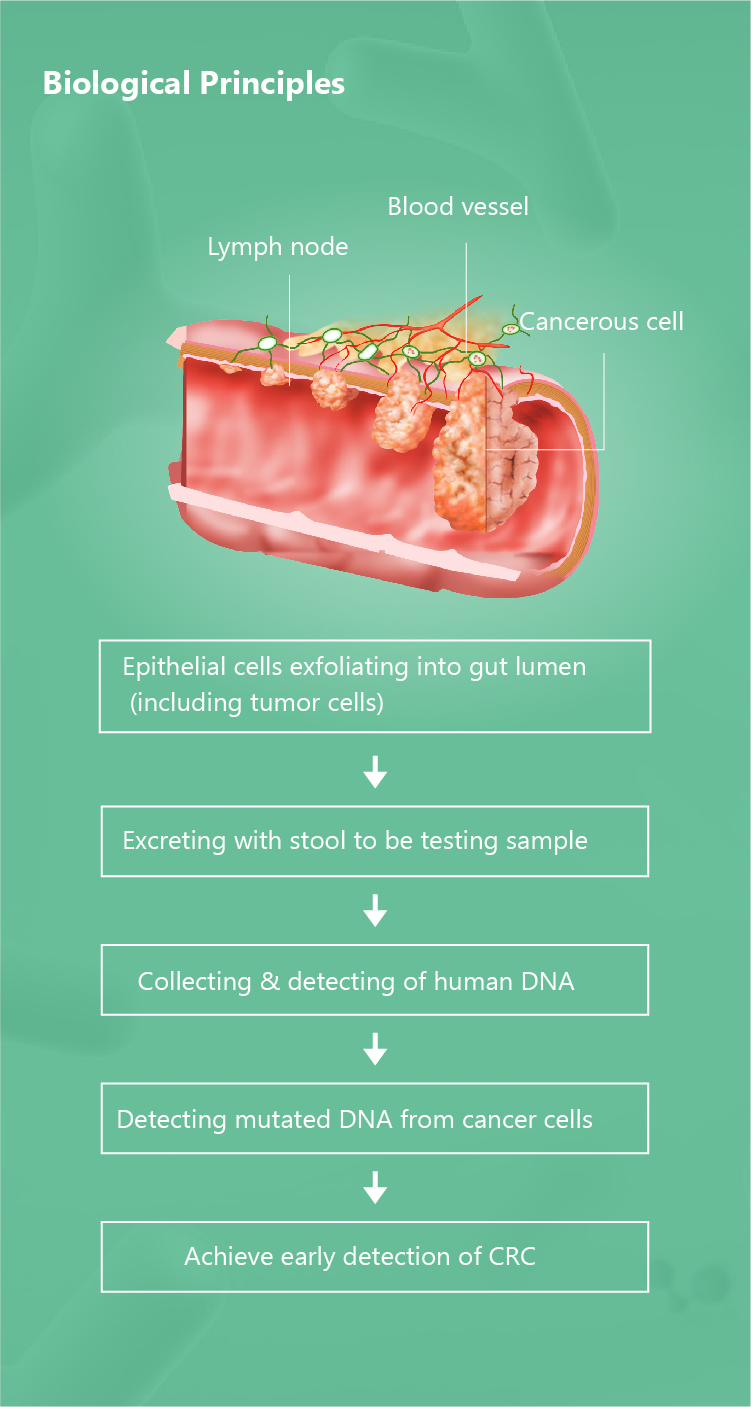
Brief Introduction of Colosafe®
Full name of Colosafe® is Methylation Detection Kit for Human SDC2 Gene (Real time PCR) & Stool
Collection Device which is the first NMPA approved stool DNA test kit for colorectal cancer.
Colosafe® entered the special approval channel of National Medical Products Administration in
March 2017 and was cleared by NMPA on Nov 20, 2018. It is a product that can precisely detect and interpret
the mutated messages (human SDC2 methylation) from stool for detection of colorectal cancer to help
physicians to figure out the cancerous lesion early and prevent the development of CRC at early stage. Thus,
the prevention and cure of CRC is achieved. It has been implemented in over 700 hospitals in China and has
gained tremendous clinical recognition by the professionals and experts.

Product Advantage
Product Performance
The sensitivity of Colosafe® to early stage colorectal cancer is 86.71%
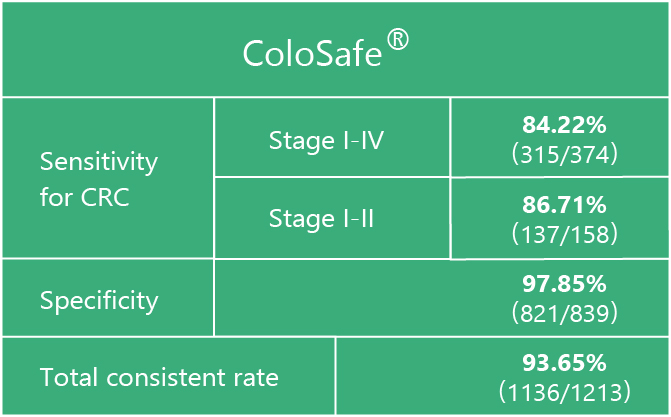
Note: n=1213,CRC=374,non-CRC=839: Kappa=0.85, Kappa>0.75, means high consistency compared with golden standards of colonoscopy and pathology.
Guideline and Publication
-

The Expert Consensus on Colorectal Cancer Screening Protocol in China (in 2019, Shanghai).
-

Robust Performance of a Novel Stool DNA Test of Methylated SDC2 for Colorectal Cancer: a Multi-center Clinical Study
-

Chinese Experts Consensus On Experimental Diagnosis of Colorectal Cancer in Precancerous Lesions and Early stage
Sample Collection Video
-
Colosafe sample collection video
Patient Stories