Creative Biosciences Successfully Completed the Acquisition of Helixgen

CreativeBio successfully acquired Helixgen in late March, 2020, marking another milestone in its burgeoning history since 2015. This significant merge will enrich the product lines bilaterally by combining CreativeBio’s strong research & development capability in the field of IVD with Helixgen’s engineering development potential. In addition, it allows Helixgen to fully utilize CreativeBio’s sales team and resource channels to promote their products. The powerful alliance of the two companies which complement each other seamlessly will definitely bring the merged enterprise up to a whole new level.

HELIXGEN, located in Guangzhou Science City, was founded in 2008. The company consists of a talented team of biologists, biomedical engineers, entrepreneurs and investors. Oriented towards research and development, it focuses on developing, manufacturing, and selling IVD products and providing related technical services. With specialists in molecular diagnostics and point-of-care testing platforms, the company is committed to make the progress in precision medicine.


Partnered with the world's leading technology team, HelixGen has recently launched HelixGenTM MHC Tetramer that can be used for immunology research, immunotherapy and vaccine development. HelixGenTM MHC Tetramer binds to antigen-specific T-cell with high sensitivity and specificity, making it possible for qualitative and quantitative analysis of T-cells, which is widely used in T-cell sorting, CTL epitope mapping, TCR affinity analysis, TCR-like antibody preparation, and CAR-T cell studies.
Helixgen is about to finish the development of POCT system as planned. Through the innovative applications of microfluidic technology in fluorescent PCR and embedded with enclosed chip set, the system is a POCT (Point-of-Care Testing, real-time detection) platform for molecular detection, an automated process that integrates sample handling, multiple PCR amplifications, DNA extractions, and the genetic analysis into a closed seamless one-time operation.



关于About Creative Biosciences (Guangzhou) Co., Ltd.
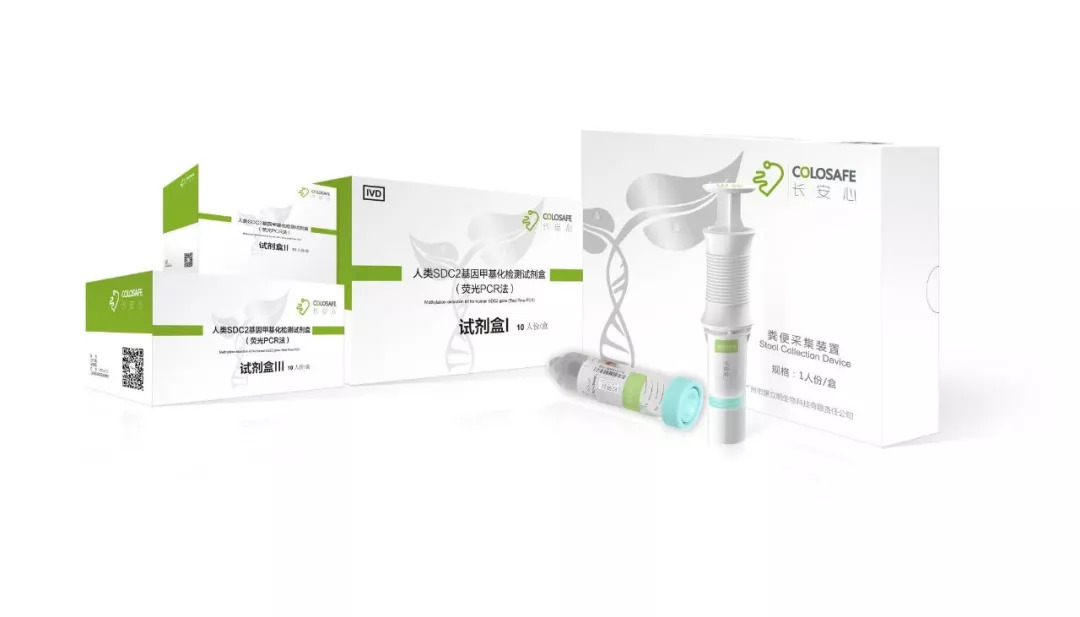
CreativeBio is a high-tech bioscience company founded in 2015 by a team of excellent scientists who had returned from abroad. It’s the proud developer, producer and seller of the top brand Colosafe-a stool DNA testing kit for early detection of colorectal cancer. The company is well known for developing detection kits for cancers as well as offering its matching testing services. Headquartered in Guangzhou, CreativeBio has set up offices in Beijing, Shanghai, Xi'an, Hangzhou and established other branches in Hong Kong and Rochester of Minnesota, USA. Its medical testing laboratories scatter nationwide in Guangzhou, Tianjin, Wuhan, Jinan and other places. As of today, the company owns a plethora of independent intellectual property rights and internationally renowned research and development capabilities. Financially, the company has completed several rounds of high-quality venture capital funding and obtained strong support at all levels from the national, provincial, municipal and district governments. It has become a star enterprise in the industry attracting extraordinary attention in China. In line with the lofty ideal of "Human Health, My Mission", we strive to achieve breakthroughs and advancements in early diagnosis, therapeutic treatment, and prognostic monitoring of cancer and other diagnostic fields for the ultimate health benefits of the people.
Colosafe is an early detection tool for colorectal cancer that can accurately interpret abnormal gene changes (human SDC2 gene methylation) in feces. It helps doctors detect patients' colorectal cancer lesions in the early stage, providing effective prevention and treatment of the disease. Colosafe (Detection Kit for Human SDC2 Gene and stool collection device) was approved by National Medical Products Administration (NMPA) on November 20, 2018 and is the only commercially available, NMPA-approved stool DNA test for colorectal cancer in China. In October 2019, Colosafe was recommended in a published expert consensus in Chinese Journal of Medicine as one of the first-line colorectal cancer screening methods. It obtained CE Mark in February 2020, allowing Colosafe to be sold in areas where CE is recognized.